New publication in "Protein Science"
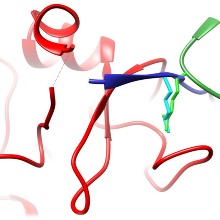
This manuscript investigates the interaction of the DNMT3A DNA methyltransferase and MECP2 methylation reader, which are both very important proteins in neurons. Both factors had been shown to interact via the DNMT3A-ADD and MECP2-TRD domains and by this regulate DNMT3A activity and subnuclear localization, but no further information about the interface was available. In this manuscript, we mapped the interface of both domains using biochemical assays and structural analyses at the level of amino acids. We show that the TRD domain binds to the ADD domain with a mechanism that resembles the well characterized H3-tail binding to the ADD domain. The interaction of MECP2 and H3 with DNMT3A is competitive and controlled by post-translational modifications.
Contact

Albert Jeltsch
Prof. Dr.Head of Biochemistry department and acting director IBTB