New publication in "Proten Science"
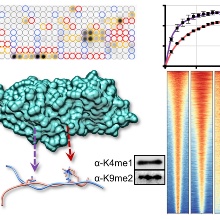
The UHRF1 protein has an essential role in the maintainance of DNA methyliaton. Despite decades of work, even the individual building blocks of this important master-regulator protein are insufficiently characterized so far. This is illustrated by our novel and totally unexpected discovery that the Tandem-Tudor domain of UHRF1 reads combined H3K4me1-K9me2/3 marks, which represents a novel as unanticipated finding. THe K4me1-K9me2/3 binding of TTD is important for the chromatin binding of full-length UHRF1 leading to UHRF1 binding near TSS and at enhancers of cell type specific genes, where it contributes to gene repression. Our biochemical work discovers a novel aspect of the UHRF1 chromatin interaction and our bioinformatic analyses document that the TTD-K4me1 interaction has a critical role in DNA methylation independent gene silencing of UHRF1. We are convinced that these findings will have an important impact on the future research on UHRF1 including its role in carcinogenesis and they will be of interest for a wide range of scientists. Moreover, our data contribute to the emerging field of combinatorial chromatin marks by the discovery that K4me1-H3K9me2/3 has a specific role in the recruitment of UHRF1 to euchromatic regions and silencing of K4me1-H3K9me2/3 marked enhancers.
Contact

Albert Jeltsch
Prof. Dr.Head of Biochemistry Department and Acting Director of the IBTB