New publication in "J. Mol. Biol."
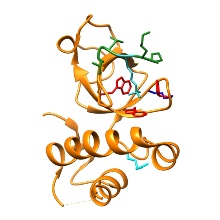
The PWWP domain of DNMT3 DNA methyltransferases binds to histone
H3 tails containing methylated K36 and this activity is important for heterochromatic targeting of
these enzymes. In this publication, we show that the PWWP domain of DNMT3A binds to H3K36me2 and
H3K36me3 with a slight preference for H3K36me2. Additionally, the domain binds to DNA with a weak
preference for AT-rich sequences. The K295I mutation recently identified in paraganglioma, a rare
neuroendocrine neoplasm, disrupts DNA and H3K36me2/3 binding. Different experiments in our study
demonstrate that the combined binding of the DNMT3A PWWP domain binds to the H3 tail containing
K36me2/3 and to the nucleosomal or linker DNA is important for its chromatin interaction and
sub-nuclear targeting of DNMT3A in living cells.