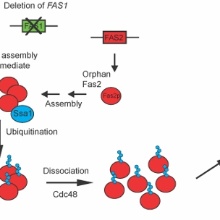
For the assembly of heterologous protein complexes in the cell the presence of stoichiometric amounts of the respective protein subunits is of utmost importance. A surplus of any of the subunits may trigger unspecific and harmful interactions and has to be avoided by the cell. Using the 2.6 Megadalton fatty acid synthase complex of yeast which is composed of two different subunits,-Fas1 and Fas2-, we can show that the absence of Fas1 leads to the selective degradation of Fas2 via the ubiquitin-proteasome system. Degradation of orphan Fas2 is triggered by the ubiquitin ligase Ubr1. The Hsp70 chaperone Ssa1 keeps the orphan Fas2 complex in a soluble form. Subsequent ubiquitination and the following action of the AAA-ATPase motor Cdc48 render orphan Fas2 to degradaion by the proteasome