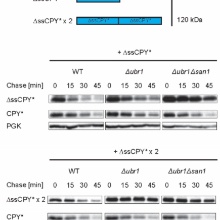
Most misfolded cytosolic proteins in the cell are eliminated by the ubiquitin-proteasome system. In yeast, polyubiquitination of misfolded cytosolic proteins is triggered mainly by the action of two ubiquitin ligases UBR1, formerly discovered as recognition component of the N-end rule pathway, and the nuclear ubiquitin ligase San1. For San1-mediated targeting to proteasomal degradation, cytosolic proteins have to be imported into the nucleus. Selection of misfolded substrates for import into the nucleus had remained elusive. This study by the group of Prof. Wolf shows that an increasing molecular mass of substrates prevents nuclear San1-triggered proteasomal degradation but renders them susceptible to cytoplasmic Ubr1-triggered degradation.