New publication in "Communications Chemistry"
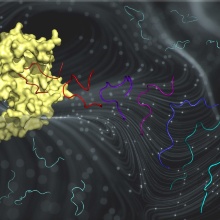
The SETD2 protein lysine methyltransferase has very important roles in chromatin regulation in human cells and strong connections to cancer. In previous work, we discovered a super-substrate of the SETD2 that is methylated about 100-fold faster than the original H3K36 substrate. However, the mechanism behind this fascinating increase in enzyme activity was not uncovered. Here, we have studied the interaction of SETD2 and the two different substrate peptides using molecular dynamics simulations and biochemical experiments and made the striking observation that the super-substrate has advantages at several steps. In solution, we show by MD and biochemical data that it prefers a hairpin conformation. Following sMD and biochemical studies then showed that this hairpin conformation binds more efficiently to SETD2. In the SETD2- peptide complex, additional contacts are formed and different transition state structures were identified for both peptides which further contribute to the increased methylation rate. Overall new principles of the substrate interaction of protein methyltransferase have been discovered that will enable numerous follow up studies.