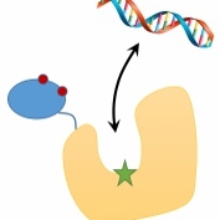
In a new paper we show that the human Dnmt1 methyltransferase is regulated by its RFTS domain acting as autoinhibitor by blocking the DNA binding pocket. We show that weakening of the interaction of the RFTS domain and the catalytic domain by mutagenesis led to a hyperactive Dnmt1 variant, which is a rare finding in mechanistic studies with enzymes. In addition, we show that this mutation also activates Dnmt1 in cells, indicating that the positioning of the RFTS domain regulates Dnmt1 activity also in cells. This process could play a role in the maintenance of DNA methlyation a central epigenetic process necessary for human embryoinc development.