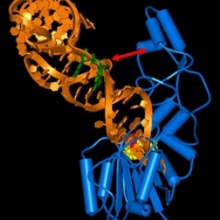
The Dnmt2 enzyme family was discovered by sequence alignments with DNA methyltransferases. Later, a seminal work showed that Dnmt2’s main substrate is tRNA-Asp (and not DNA), a finding that has been confirmed for Dnmt2 enzymes from various species. We now show that unexpectedly Geobacter Dnmt2 preferentially methylates Geobacter tRNA-Glu, and not tRNA-Asp, indicating a swap of substrate specificity. We put this finding in the context of the acquisition of Dnmt2 by Geobacter by horizontal gene transfer. Based on the sequences of the known substrate and non-substrate tRNA of Geobacter Dnmt2, we could identify that the variable loop plays an important role in tRNA recognition of Dnmt2 (indicated by the red arrow in the figure). Interestingly, it turned out that the same recognition process is functional in the human DNMT2 enzyme, such that our study of a bacterial enzyme has led to the identification of a previously unknown principle of tRNA recognition by the human Dnmt2, which will certainly help understanding the function of this enzyme.